[ad_1] A multimillion-dollar legal case levelled at British pharmaceutical giant AstraZeneca has claimed the Oxford-AstraZeneca Covid-19 vaccine adm
[ad_1]
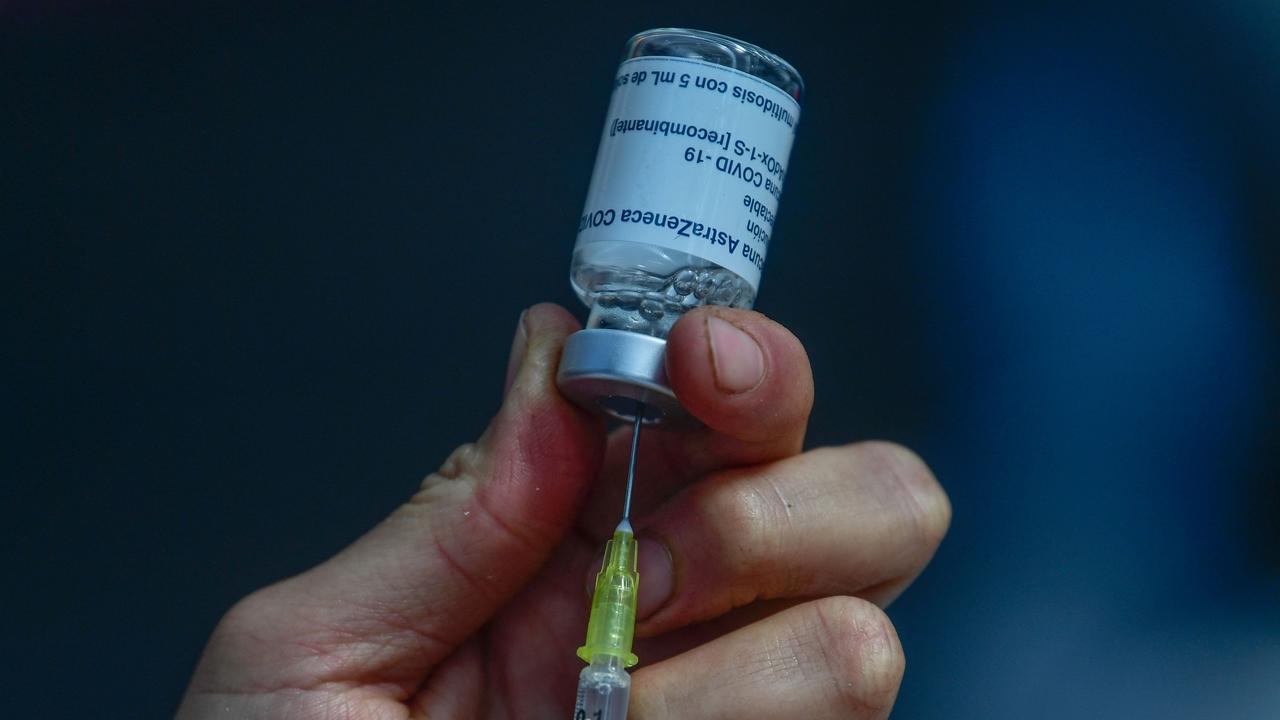
A multimillion-dollar legal case levelled at British pharmaceutical giant AstraZeneca has claimed the Oxford-AstraZeneca Covid-19 vaccine administered in millions of people was “defective”.
The landmark High Court case was initiated by UK father Jamie Scott, who suffered a permanent brain injury from a blood clot after receiving the vaccine in April 2021.
Another claim has been brought by the widower of Alpa Tailor, who died after developing blood clots on her brain post-vaccination. The 35-year-old mother said she had chosen to get the jab to “protect her family”.
The legal action could lead to as many as 80 damages claims totalling £80 million (A$153 million) over Vaccine-induced Immune Thrombocytopenia and Thrombosis (VITT), a condition identified after the worldwide vaccine rollout.
Independent studies claimed the vaccine saved over six million lives globally in its first year, but concerns over rare side effects led to its discontinuation in the UK and Australia.
AstraZeneca claims it shipped two billion doses of its vaccine across the world after its initial approval.
But in May 2021, the UK’s Joint Committee on Vaccination and Immunisation advised those under 40 with no underlying health conditions to steer clear.
In 2022, the World Health Organisation declared the vaccine was “safe and effective for all individuals aged 18 and above” and that adverse events were “very rare”.
The AstraZeneca Covid vaccine, sold under the brand name Vaxzevria, has not been available to the Australian public since March 20, 2023.
AstraZeneca said the safety of patients is its “highest priority” and insisted its Covid-19 vaccine had “continuously been shown to have an acceptable safety profile”.
But lawyers are arguing that the information in the AstraZeneca press release on its efficacy was “misleading because members of the public … assume that the published efficacy rate was an absolute risk rate (in which case the published efficacy rate vastly overstated the efficacy of the vaccine)”.
According to the Medicines and Healthcare products Regulatory Agency, at least 81 deaths in the UK are suspected to be linked to adverse reactions causing clotting in individuals with low blood platelets.
The case will also put under a microscope the UK Government’s decision to grant indemnity for AstraZeneca in the “very unexpected event of any adverse reactions that could not have been foreseen through the robust checks and procedures that have been put in place”.
Lawyers noted that the Secretary of State for Health and Social Care Matt Hancock, in an accompanying departmental minute, said: “The data so far on this vaccine suggests that there will be no adverse reactions, and so no liability.”
The Court documents state: “The Claimant claims damages and interest … as a result of personal injuries and consequential losses arising out of his sustaining Vaccine Induced Immune Thrombosis with Thrombocytopenia (VITT) as a result of his vaccination on 23 April 2021, with the AstraZeneca COVID-19 vaccination (ChAdOx1-S [recombinant]) manufactured and/or supplied by the Defendant which was defective within the meaning of the Consumer Protection Act 1987.”
Father of two forced to quit job
Mr Scott said he had to give up his job as an IT software developer as a result of the side effects.
“We are private people but we cannot stand the injustice of it. We have been lobbying the Government for 18 months for fair compensation for the injury caused by the vaccine,” Mrs Scott said via the UK Telegraph.
“We were told by the Government the vaccine was safe and effective but what’s happened to Jamie has been life changing and their [AstraZeneca] vaccine caused that.”
“AstraZeneca cannot continue to ignore the circumstances in which their vaccine has caused devastating injury and loss. Our legal case will seek to hold AstraZeneca to account but we need to build a significant fighting fund to get justice.”
AstraZeneca’s own press releases following clinical trials said the vaccine was between 62 per cent and 90 per cent effective at preventing symptomatic Covid-19 depending on dosages, with an average of 70 per cent.
“In fact, the absolute risk reduction concerning Covid-19 prevention was only 1.2 per cent,” the legal claim stated.
Australia’s Therapeutic Goods Administration (TGA) provisionally approved the AstraZeneca vaccine for use in Australia for people aged 18 years and over as a primary course from February 15, 2021 and as a booster from February 8, 2022.
At the time, the TGA said the decision to receive a Vaxzevria booster must be made in consultation with a health professional and that mRNA Covid vaccines (such as Pfizer and Moderna’s) were “preferred” boosters.
Months later, however, medical experts started to recommend against Australians under 60 taking the AstraZeneca vaccine due to concerns over a potentially-fatal blood clotting disorder: Thrombosis with thrombocytopenia syndrome (TTS).
The change came after a number of cases of TSS among those aged 50-59 years, and the death of a 52-year-old woman from a blood clot likely linked to the AstraZeneca vaccine.
The risk of developing one was slightly higher in younger patients.
A poll conducted by news.com.au in 2022 showed more than half of the respondents regret getting vaccinated, or were unvaccinated and happy with their decision.
Only 35 per cent out of more than 45,000 people said they were vaccinated and would make the same decision again.
AstraZeneca profit almost doubles despite Covid slump
Profits are still rising for the pharmaceutical giant despite facing massive legal battles.
AstraZeneca said Thursday that third-quarter net profit nearly doubled on solid demand for cancer treatments, despite evaporating sales of Covid medicines.
Net profit soared almost 90 per cent to $1.26 billion in the three months to the end of September compared with a year earlier, the Covid-vaccine maker said in a statement.
Total revenues gained five per cent to $33.8 billion despite a $2.9-billion slump in revenue from Covid treatments.
The group was boosted by a 20-percent jump in sales of oncology drugs.
“Our company continued its strong growth trajectory in the third quarter,” said chief executive Pascal Soriot.
While overall sales grew, revenue from its Covid jab Vaxzevria slumped 98 per cent in the first nine months of the year compared with the same part of 2022.
AstraZeneca also lifted its annual revenue guidance, sending its share price climbing 2.8 per cent to almost £105 (A$201) in morning deals on London’s top-tier FTSE 100 index.
[ad_2]
Source link


COMMENTS